Why Is Bromine A Liquid At Room Temperature And Chlorine A Gas

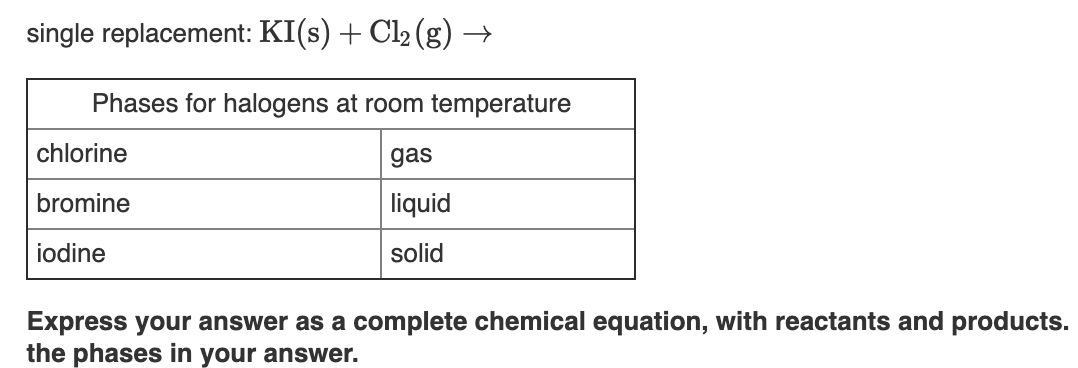
At this temperature fluorine and chlorine are gases bromine is a liquid and iodine and astatine are solids.
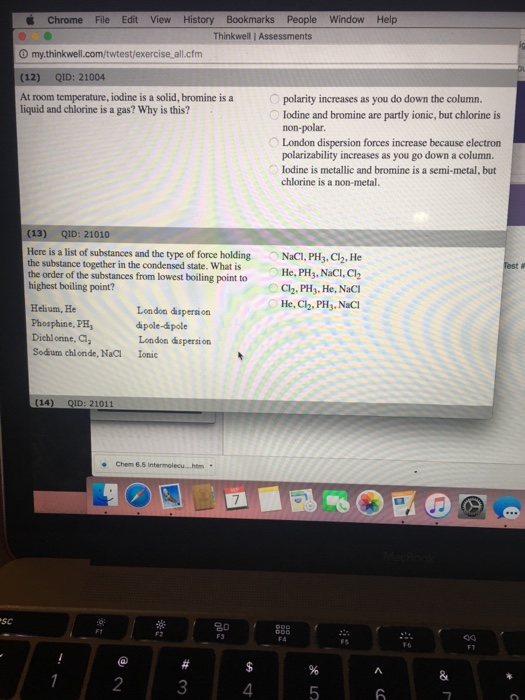
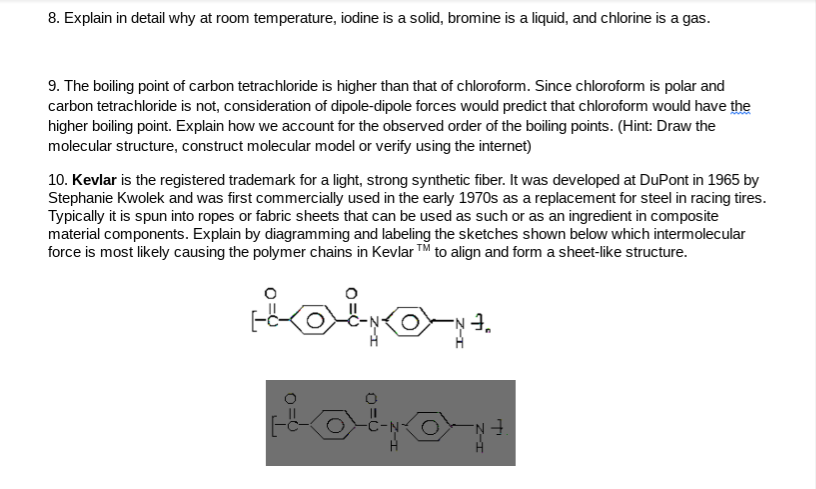
Why is bromine a liquid at room temperature and chlorine a gas. It is the third lightest halogen and is a fuming red brown liquid at room temperature that evaporates readily to form a similarly coloured gas. Chlorine is a gas at room temperature but bromine is a liquid and carbon is a solid. Why is bromine liquid at room temperature. At room temperature iodine is a solid bromine is a liquid and chlorine is a gas.
Bromine is a naturally occurring element that is a liquid at room temperature. London dispersion forces increase because electron polarizability increases as you go down a column. Where bromine is found and how it is used. Iodine and bromine are partly ionic but chlorine is non polar.
What bromine is. Explain why at room temperature fluorine and chlorine are gases bromine is a liquid and iodine is a solid. It has a brownish red color with a bleach like odor and it dissolves in water. Its properties are thus intermediate between those of chlorine and iodine isolated independently by two chemists carl jacob löwig in 1825 and antoine jérôme balard in 1826.
Bromine is a chemical element with the symbol br and atomic number 35. Room temperature is usually taken as being 25 c. Since bromine molecules have more than twice the mass of chlorine molecules they tend to stick to each other more than chlorine molecules do and are more likely to be a liquid at room temperature while chlorine molecules are more likely to be a gas at the same temperature. Polarity increases as you do down the column.
Bromine is found naturally in the earth s crust and in seawater in various chemical forms.